valence electrons argon|Iba pa : Pilipinas The total number of electrons in the last shell after the electron configuration of argon is called the valence electrons of argon. The last shell of argon has eight . University of Perpetual Help System JONELTA: Binan • GMA • Manila • Pangasinan • Isabela • Roxas City • UPH-DJGTMU: Home. Programs Graduate School Athletics Arts and Sciences Business and Accountancy Computer Studies Criminology Education Engineering and Aviation Int'l Hospitality Management Maritime Juris Doctor Senior High School .
PH0 · valence electrons for kids
PH1 · valence electrons chart
PH2 · valence electrons calculator
PH3 · transition metals valence electrons list
PH4 · number of valence electrons fe
PH5 · list of valence electrons for each element
PH6 · how do you find valence electrons
PH7 · finding valence electrons in ions
PH8 · Iba pa
At Friends Indeed, we believe that every dog deserves love, care, and a forever home. Our team of passionate volunteers works tirelessly to rescue dogs from challenging situations, ensuring their.You can contact @PINAYRARE_CHANNELBOT right away. . If you have Telegram, you can contact PINAY RARE BOT CHANNEL right away.
valence electrons argon*******Mar 23, 2023
The total number of electrons in the last shell after the electron configuration of argon is called the valence electrons of argon. The last shell of argon has eight . You may assume the valences of the chemical elements—the number of . Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2 s subshell and four in the 2 p subshell. We can write the configuration of oxygen's valence . Well, Argon has an absolute zero valency. Argon holds 18 electrons in its outer shell with the electron configuration of 2,8,8. So, this is why Argon has no need to . The total number of electrons present in the valence shell of an atom is called valence electrons, and there are eight electrons present in the valence shell of argon (3s²3p⁶). Thus, argon has eight . Valence electrons are the outer-shell electrons of an atom. Valence electrons determine the reactivity of an atom. Atoms have a tendency to have eight .Iba paAny element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons). Examples include neon (Ne), argon (Ar), and krypton (Kr). Oxygen, . Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. .valence electrons argon The electron configuration of argon is [ Ne] 3s 2 3p 6 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Electron configuration through orbit (Bohr principle) . The \(1s\) electrons in oxygen do not participate in bonding (i.e., chemistry) and are called core electrons. The valence electrons (i.e., the \(2s^22p^4\) part) are valence electrons, which do participate in the making and breaking of bonds. Similarly, in calcium (Equation \(\ref{3}\)), the electrons in the argon-like closed shell are the core .
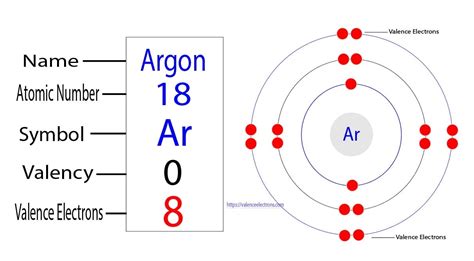
Thus, argon has eight valence electrons. Valency of Argon (Ar) There are many different ways to find out the valency of an atom which reflects the ability of an atom to bond with other atoms. . Now, the electron configuration of argon shows that the last orbit has eight electrons. Therefore, the valence electrons of argon are eight. The last shell of argon has no unpaired electron, so the . For example, fluorine has seven valence electrons, so it is most likely to gain one electron to form an ion with a 1- charge. We can use this method to predict the charges of ions in ionic . There are two ways to find the number of valence electrons in Argon (Ar). The first is to use the Periodic Table to figure out how many electrons Argon has i.
sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group .
Examples include hydrogen (H), lithium (Li), and sodium (Na). Any element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons). Examples include neon (Ne), argon (Ar), and krypton (Kr). Oxygen, like all the other elements in group 16, has six valence electrons. Discuss further with Flexi.

This periodic table shows the valences of element groups. The transition metals make use of the d-subshell, which can accommodate 10 electrons.The f-subshell holds 14 electrons and the g-subshell contains up to 18 electrons.Metals in the middle of the periodic table become more stable by emptying a shell, half-filling it, or completely .
E-sports Online Betting Singapore. Get in on the action with Playdash’s e-sports betting, where the digital arena offers limitless opportunities to win. Bet on popular games like League of Legends, Dota 2, and CSwith diverse markets that include match winners, map results, and player performance. .
valence electrons argon|Iba pa